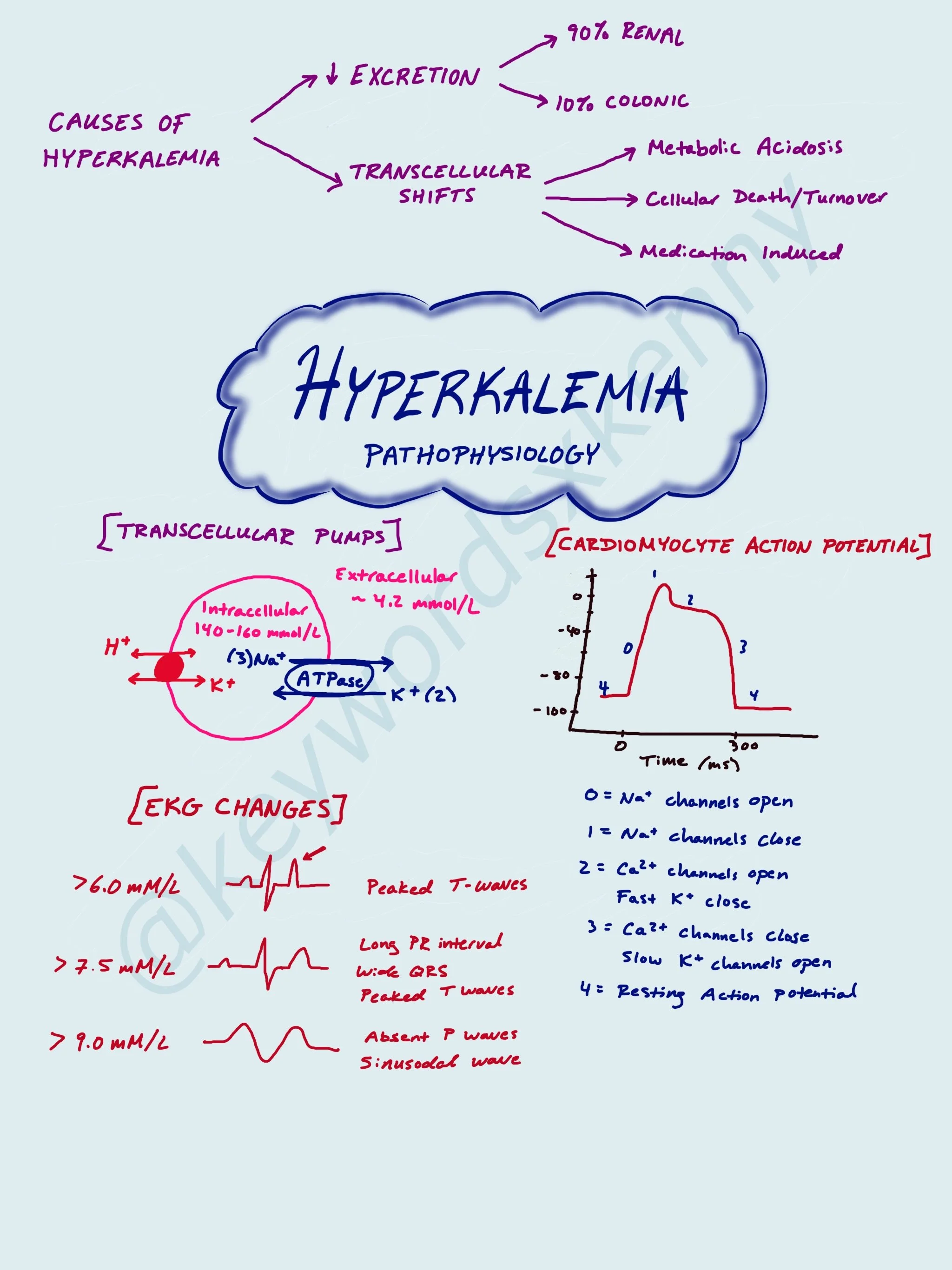
Pathophysiology of Hyperkalemia
Potassium is one of the most important electrolytes circulating in the body, having effects on multiple organ systems. Cells within our body create a perfect balance of storing majority of the potassium within the cell and allowing a regulated amount to be circulating plasma volume. While many patients in the hospital tend to need supplemental potassium for low levels (hypokalemia), this post is going to be covering what happens when patients have high levels of potassium (hyperkalemia). Hyperkalemia can be fatal if not recognized and addressed quickly enough. The reason it is so deadly is because of its effect on cardiac cells (cardiomyocytes) and potential for fatal arrhythmias. In fact, a potassium-rich solution (cardioplegia) is used during cardiac surgery to stop the heart from beating during cardiopulmonary bypass.
While there are a multitude of reasons why a patient may become hyperkalemic, let’s discuss 3 main reasons we tend to see it in the perioperative world:
1: Defective K+ Excretion
2: Transcellular K+ Shifts
In terms of potassium excretion, there are two primary ways the body eliminates it: via the renal (90%) and colonic (10%) systems. If patients are unable to urinate (ie. Renal failure requiring dialysis) there is a risk for developing hyperkalemia.
Like I mentioned before, majority of the body’s potassium is stored within cells. Potassium shifting outside of the cell is induced by metabolic acidosis and cellular death. Two clear examples of these scenarios are hyperkalemia in patients with sepsis or DKA and patients with tumor lysis syndrome or rhabdomyolysis. The factors that shift potassium back into cells are insulin and beta-adrenergic signaling.
One specific cause of intracellular K+ release that we see in anesthesia is succinylcholine-induced hyperkalemia. Succinylcholine is often the paralytic of choice when performing a rapid sequence induction (RSI) or breaking laryngospasm because of its rapid onset of action. For most patients, you can expect a rise of 0.5 mEq/L in the serum potassium. Most of the time this is a negligible rise if the patient has a normal potassium level at baseline. However, if the patient has an elevated K+, succinylcholine may not be the ideal paralytic. There are also patients who will have a more profound increase in serum potassium after Succinylcholine administration. These populations include patients with severe burns, spinal cord injuries, and muscular dystrophies. This is because these patients have “extra-junctional” acetylcholine receptors (AChR), which are immature-AChRs that grow outside of the neuromuscular junction. Succinylcholine will bind to these receptors, as well as the junctional receptors, and cause a larger release of intracellular K+ into the patient’s circulating plasma.
Now let’s talk about the cardiac changes on EKG that you can see with hyperkalemia. Cardiomyocytes have a resting action potential of -90 mV (polarized). As the concentration of potassium in circulating plasma increases, it creates depolarization of the resting potential (moving it closer to neutral) and closer to the threshold for fast-acting sodium (Na+) channels to be activated and opened. This is commonly seen at T-wave peaks on EKG as a large portion of ventricular cardiomyocytes undergo early repolarization. In cases of severe hyperkalemia, there is voltage-depended inactivation of fast-acting Na+ channels and activation of K+ channels leading to reduction in conduction velocity and can leave cardiomyocytes refractory to excitation. This can be seen on EKG as broadening QS complexes and eventually a sinusodal pattern.